The New UK Cannabinoid Framework
We explore the recommendations in the Prime Minister’s Taskforce on Innovation, Growth and Regulatory Reform (TIGRR) report
Our review of the recent TIGGR report is submitted by
Dr Parveen Bhatarah | Regulatory and Compliance Associate at The Centre for Medicinal Cannabis
The much-needed regulatory reform proposals for this fast-moving global cannabinoid industry are here in the UK to capture the full value of today’s cannabinoids market, and position itself to profit from the future high-value innovations that will emerge in response to investment.
The recommendations in the Prime Minister’s Taskforce on Innovation, Growth and Regulatory Reform (TIGRR) report demonstrate the huge opportunities in the UK to lead the cannabinoid industry globally on standards, encourage an approach which ensures that regulatory systems are fit for the future economy, and have the capability to adapt to innovations in the technology sector. Facilitating competition and encouraging new market entrants is at the heart of these regulations.
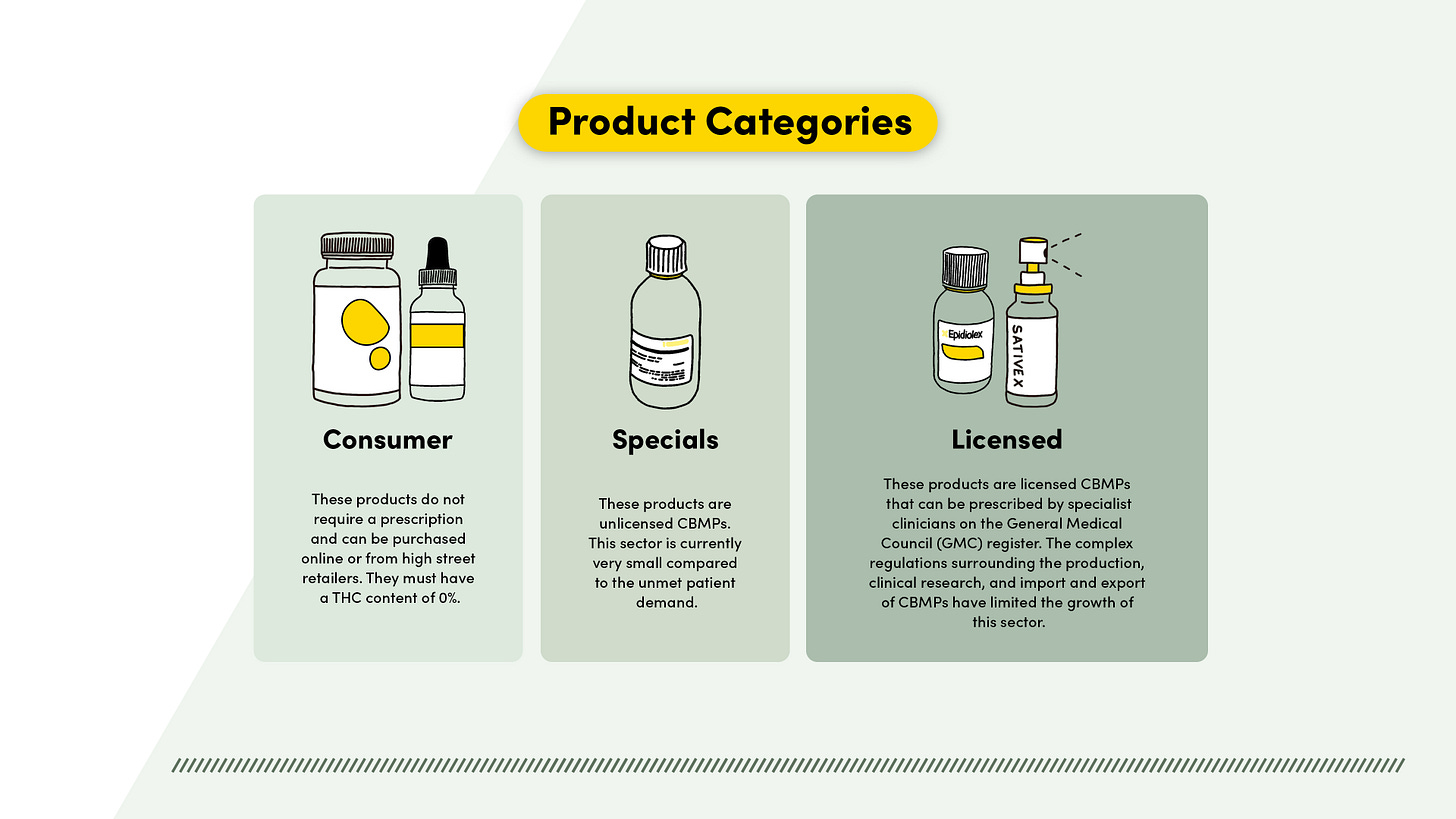
The TIGRR report gives all the necessary strategic drivers from Innovation and R&D to the genetics and agricultural sectors; this covers the entire supply chain for Medicinal Cannabinoids, Specials and Nutraceuticals. However, the recommendations only recommend legal-to-use CBD medicinal products and does not recommend decriminalisation for recreational use.
The proposal 11.15: “Regulations of medical cannabinoids and medicinal CBD should move from Home office to DHSC/MHRA” is testimony to the fact that the current difficulties which are faced by pharmaceutical scientists are a deterrent to innovation, R&D and significant investment in the cannabinoid sector in UK. However, these proposals aspire to separate medical benefits from the criminal associations and abuse potential.
The difficult licensing requirements so far have suppressed R&D in this sector and resulted in a lack of much needed skill sets to handle these cannabinoids. These proposals therefore promote the goal of a single, over-arching strategy for the UK cannabinoid sector, which would cover the whole range of scientific, commercial, and industrial activities involving hemp and cannabinoids, and give the country a competitive advantage.
These proposals in the cannabinoid sector will encourage the industry to take steps to influence the UK’s underlying strengths, the sector can potentially support many more jobs and promote direct investment into the economy that needs it, especially after Brexit and COVID: namely agriculture, manufacturing, life sciences, and technology-led innovation. If a coherent strategy is adopted across a range of sectors, they will reinforce other Scientific and Environmental goals, including Net Zero.
The TIGRR report confirms that the New post-Brexit UK is ready to remove the unnecessary regulatory burden and this shift is already evident in the Advisory Council on the Misuse of Drugs (ACMD) consultation issued in March 2021 and the Home Office intention (made explicit in the letter from the Minister of State 2021, Gov.uk, retrieved from: Advice on consumer CBD (cannabidiol) products - GOV.UK (www.gov.uk)) to seek a new settlement that will regularise the large grey market in consumer CBD products and provide legal clarity for the wider industry.
Such moves to regulate the sector by the Home Office and Food Standards Agency (FSA) are only underway now however, the proposal to move from Home Office to Department of Health and Social Care (DHSC) and Medicines and Healthcare products Regulatory Agency (MHRA) will further catalyse the acceleration of these regulatory reforms.
Overall, the TIGRR proposal is covering the comprehensive Cannabinoid strategy as proposed in ‘Green Shoots - Sowing the seeds of the new UK cannabinoid market’ for Medicines, Nutraceuticals, Environment R&D and Technology.
Medicines – Moving regulatory control of medicinal cannabinoids & medicinal CBD to DHSC/MHRA will assist in more manufacturing, research, and clinical trials for patient benefit. The further proposals related to optimising and modernising the clinical trials environment will assist in real world data gathering and approval of Cannabinoid medicine.
Furthermore, the proposal on digital health technology regulatory & medical devices reforms will offer prospects to quickly identify high valued opportunities and routes to market. This will foster new biotech companies/big Pharma wanting to exploit cannabinoid medicine for new end-indications.
Nutraceuticals – These regulatory reforms will assist in setting provenance in UK derived supply chain for consumer cannabinoid from fresh biomass to extracted and developed finish products in the UK (ensuring quality and traceability) and opportunity to export this “gold standard” to foreign markets. Phyto-cannabinoids are creating a new sector for health enhancing ‘superfoods’ and supplements. Traditional regulatory framework with binary separation of medicines (MHRA) and food (FSA) does not fit well as we discovered during the Novel Food’s dossier submission. New proposed regulatory reforms should help to clarify this grey area or establish a new regulatory pathway to allow this sector to reach its potential.
Environment – Proposal to replace EU-rules with an integrated agri-environment framework which better supports development means that these regulations offer an opportunity to develop new hemp strains & use innovative gene editing & genetic engineering to create framework encouraging biodiversity. This represents an opportunity both for the UK’s domestic production and in exporting technology.
R&D and Technology – All the proposed regulatory reforms encourage new agri-tech, MedTech & AI innovations. These innovations create new opportunities and the UK can be the source for cannabinoid standards. The TIGRR report proposes that where possible regulatory reforms should focus on the outcomes rather than the inputs; regulating the end products not the process and hence recommendations on stronger duties for regulators to promote innovation, competition, pushing them to play a much more active role in supporting growth while still maintaining the highest regulatory standards in relation to both food and the environment.
The proposal that the UK needs a Centre of Excellence in Regulatory Science and Innovation (CERSI) network, is a sensible option for MHRA to work with stakeholders to establish a UK Regulatory Innovation Hub and is an excellent way to establish a sustainable, compliant & safe Cannabinoid Industry.
The UK has been the largest importer of cannabis for use in the medicinal and consumer cannabinoids industry and the market size of the UK’s consumer CBD market is predicted to be £690m in 2021. However, once these proposed regulatory reforms are implemented it will further assist in the significant growth of this sector and the effects will be seen across the whole economy.
A New innovative cannabinoid regulatory framework will be a major boost to both UK cannabinoid industry and long-term competitiveness.